Polyostotic fibrous dysplasia MRI
Polyostotic fibrous dysplasia is a rare bone disorder where normal bone and bone marrow are replaced by fibrous tissue, leading to structurally weak, misshapen, and brittle bones. This condition is polyostotic when it affects multiple bones in the body, distinguishing it from monostotic fibrous dysplasia, which affects only one bone. It is one of the manifestations of the McCune-Albright syndrome but can also occur in isolation.
Causes
Polyostotic fibrous dysplasia is caused by a mutation in the GNAS gene, which encodes a protein involved in the regulation of cyclic AMP (cAMP) in cells. This mutation occurs after conception, in the early stages of fetal development (a post-zygotic mutation), meaning it is not inherited from the parents but occurs sporadically. The mutation leads to overactivity of bone-forming cells (osteoblasts), causing the abnormal bone growth and lesions characteristic of the disease.
Symptoms
Symptoms of polyostotic fibrous dysplasia vary depending on the number and location of bones involved but typically include:
- Bone pain and deformities
- Bone fractures
- Uneven growth of limbs
- Scoliosis or other spinal deformities
- Skin pigmentation (café-au-lait spots), especially when part of McCune-Albright syndrome
Diagnosis
Diagnosing polyostotic fibrous dysplasia usually involves a combination of clinical examination, imaging, and possibly genetic testing:
- X-rays: Show characteristic changes in bone, such as irregular, cyst-like areas or “ground glass” opacity.
- Bone scan: Helps in identifying the extent and activity of the disease.
- MRI or CT scans: Provide detailed images of bone and can help in assessing the extent of bone deformity and planning surgical interventions.
- Biopsy of bone tissue: Confirms the diagnosis by showing the replacement of normal bone with fibrous tissue and abnormal bone.
- Genetic testing: Can identify mutations in the GNAS gene, especially useful in cases where the diagnosis is uncertain.
Treatment
Treatment for polyostotic fibrous dysplasia is usually symptomatic and aimed at managing pain, preventing fractures, and correcting deformities:
- Medication: Bisphosphonates can help reduce bone pain by decreasing bone turnover.
- Surgery: May be necessary to correct bone deformities, fix fractures, or stabilize bones at risk of fracturing.
- Pain management: Includes the use of analgesics and anti-inflammatory drugs.
- Hormonal therapy: If the disease is part of McCune-Albright syndrome, endocrine abnormalities such as precocious puberty or hyperthyroidism may need treatment.
- Physical therapy: Helps strengthen muscles around affected bones and improve mobility.
MRI Appearance of Polyostotic Fibrous Dysplasia of Humerus
MRI T1 Appearance of Polyostotic Fibrous Dysplasia
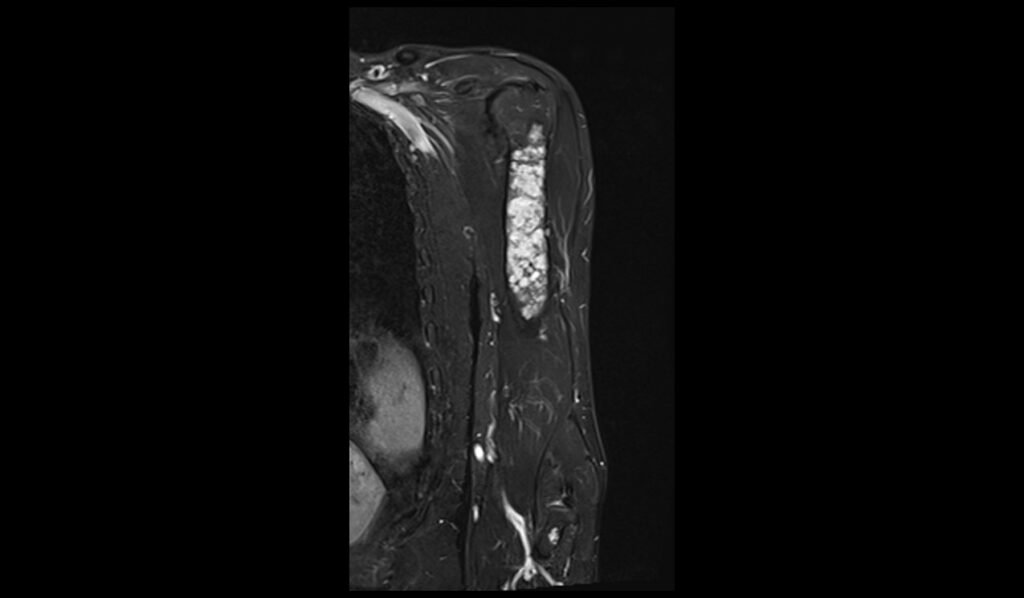
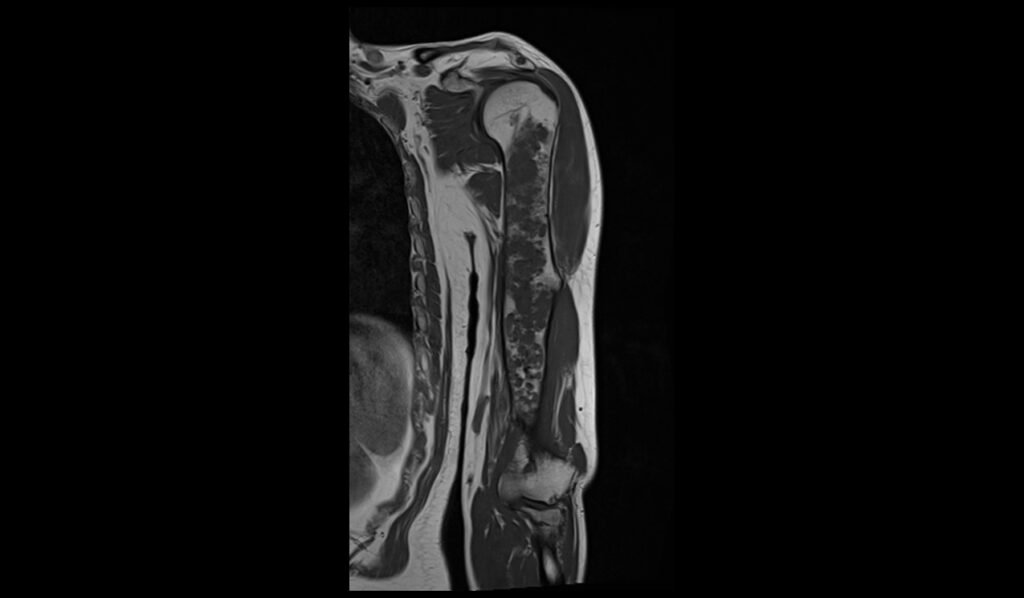
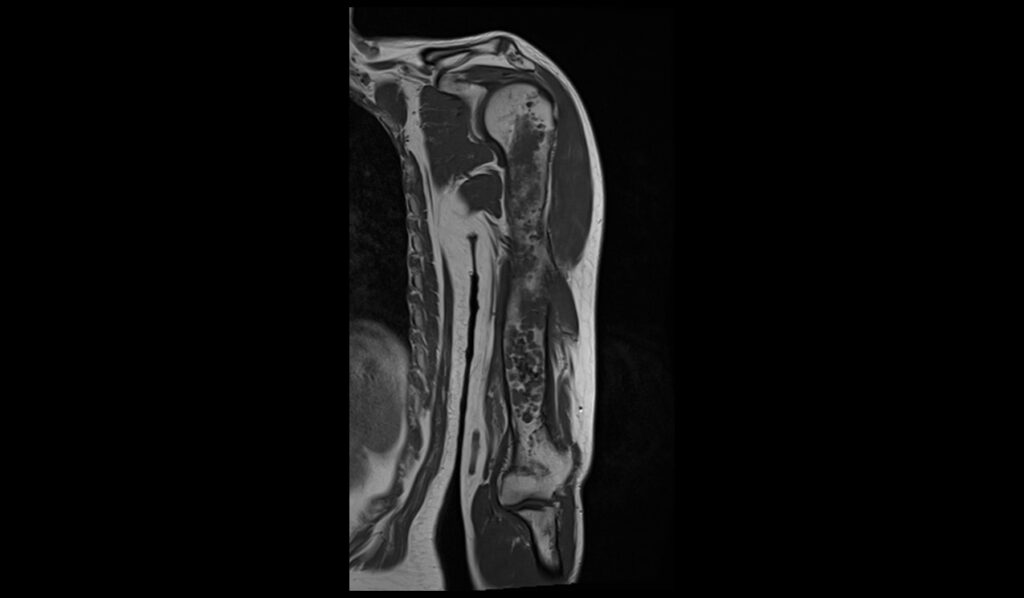
In T1-weighted MRI images, polyostotic fibrous dysplasia typically presents with low to intermediate signal intensity compared to normal bone marrow. The affected areas of bone often appear hypointense due to the replacement of normal bone marrow with fibrous tissue. This hypointense signal is relatively uniform, though there may be some areas of slightly higher intensity if there is associated hemorrhage or cystic changes within the lesions. The borders of the lesions are usually well-defined but can sometimes appear irregular due to the expansive nature of the fibrous tissue.
MRI T2 Appearance of Polyostotic Fibrous Dysplasia
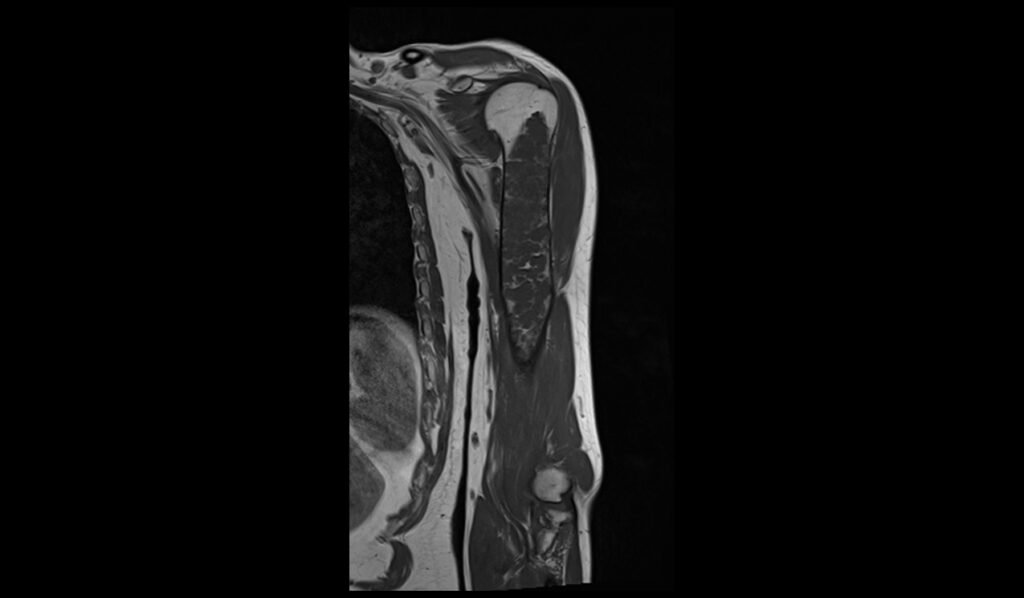
On T2-weighted MRI images, polyostotic fibrous dysplasia typically shows a heterogeneous appearance with variable signal intensity. The fibrous tissue within the dysplastic bone lesions generally appears hyperintense compared to normal bone marrow, reflecting the increased water content of the fibrous tissue. This hyperintensity can be interspersed with areas of hypointensity if there are calcifications or sclerotic regions within the fibrous dysplasia. The lesions often show a mix of these signal intensities, giving a characteristic “ground-glass” appearance, and the overall pattern is quite distinctive.
MRI STIR Appearance of Polyostotic Fibrous Dysplasia
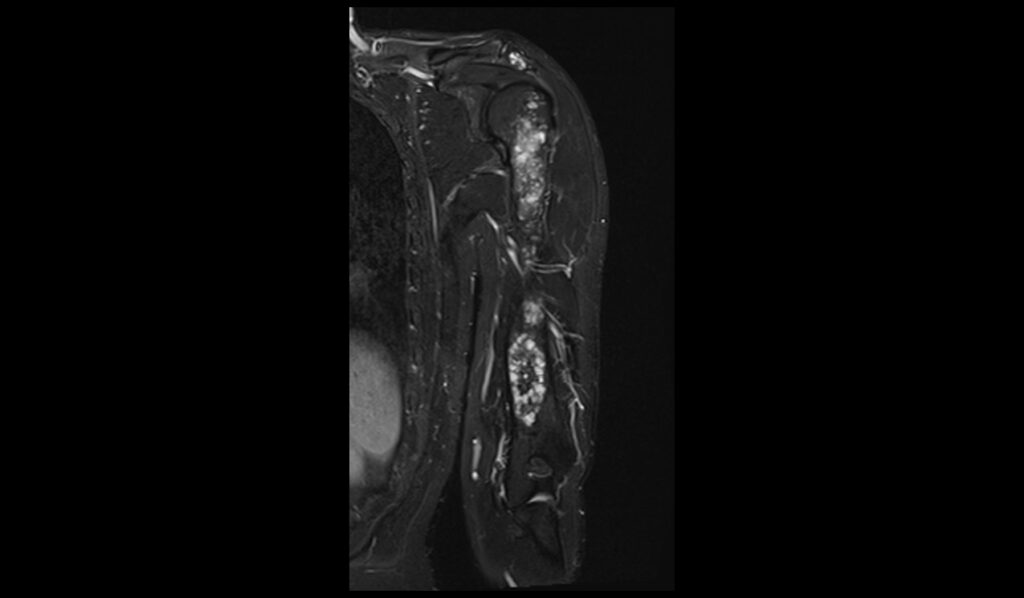
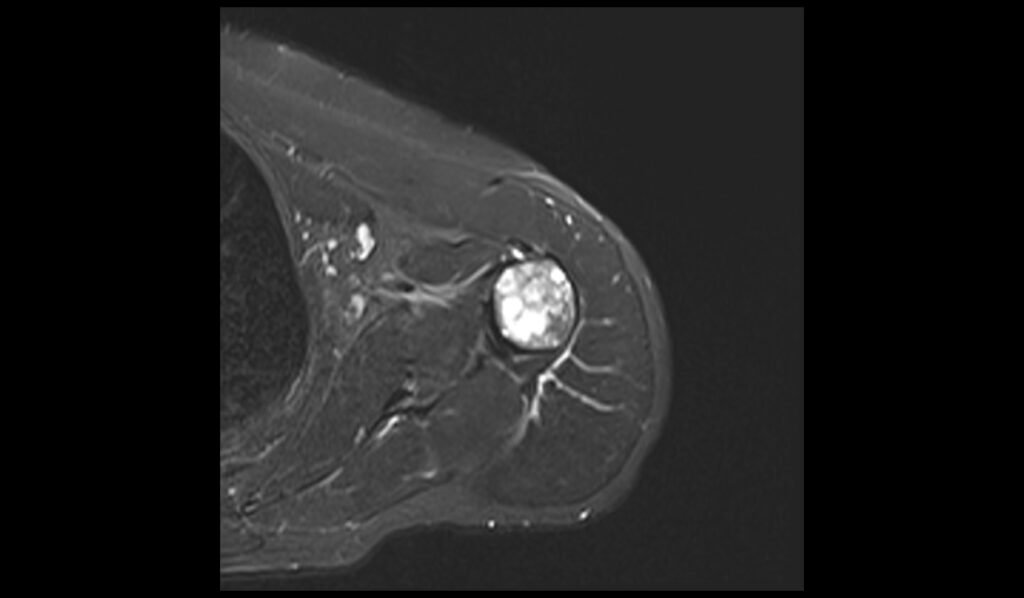
Short Tau Inversion Recovery (STIR) sequences in MRI are particularly sensitive to the presence of edema and high water content tissues. In polyostotic fibrous dysplasia, STIR images typically reveal markedly hyperintense lesions due to the high water content in the fibrous tissue. This sequence is excellent for highlighting the extent of the fibrous dysplasia, as the lesions stand out brightly against the darker background of normal bone marrow. The increased signal intensity on STIR images helps in assessing the full extent of bone involvement and any associated inflammatory changes, making it a valuable tool in the evaluation of this condition.
STIR coronal image shows Humerus Polyostotic Fibrous Dysplasia
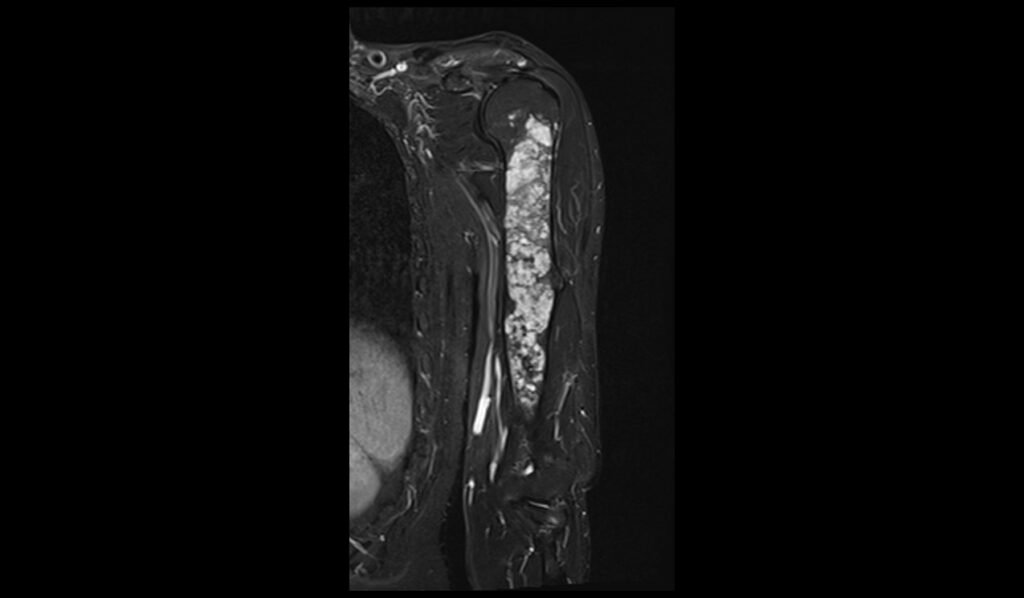
T1 TSE coronal image shows Humerus Polyostotic Fibrous Dysplasia
STIR axial image shows Humerus Polyostotic Fibrous Dysplasia
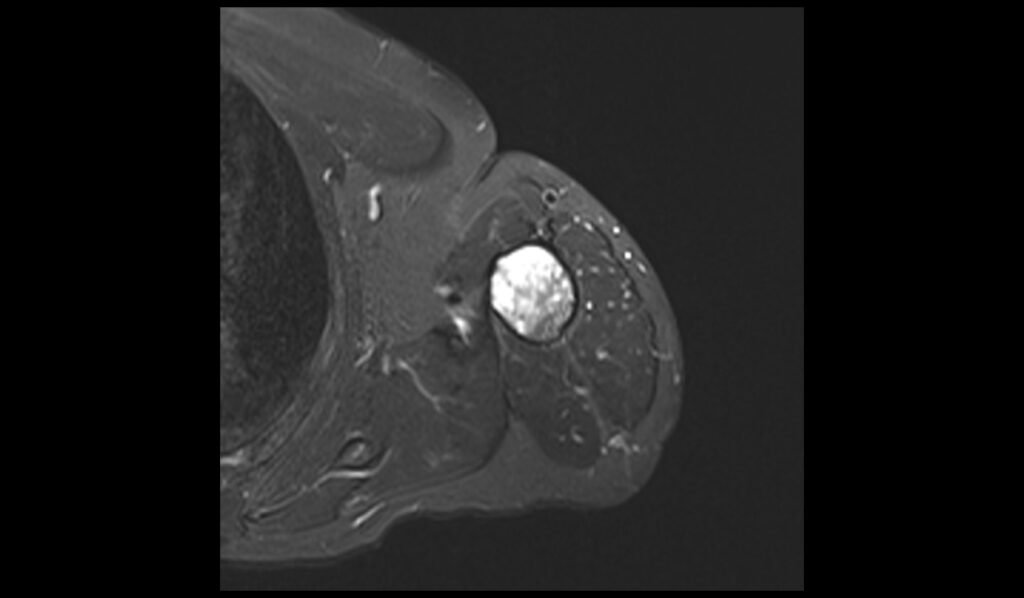
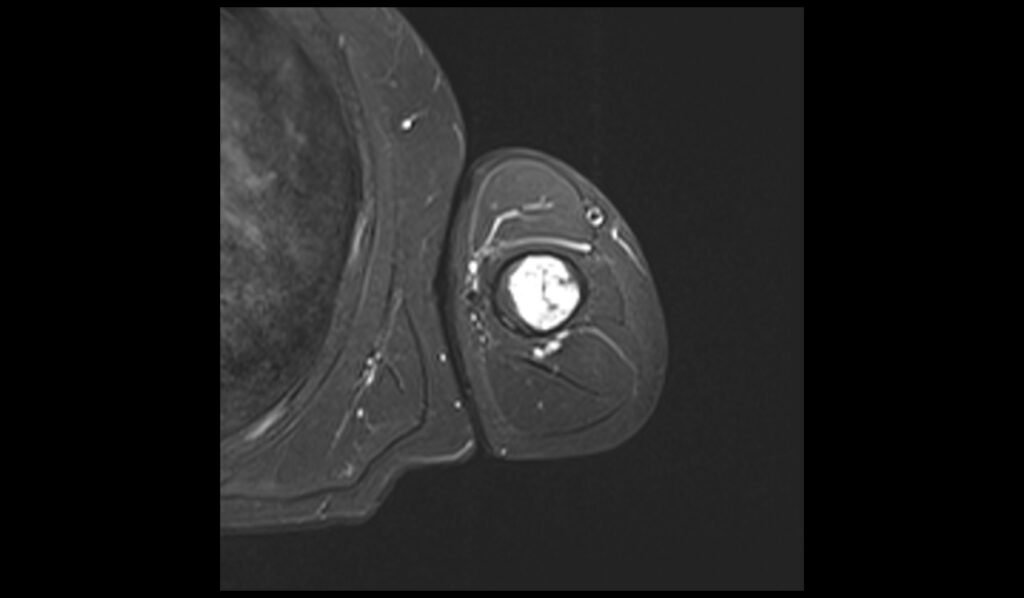
T1 axial image shows Humerus Polyostotic Fibrous Dysplasia
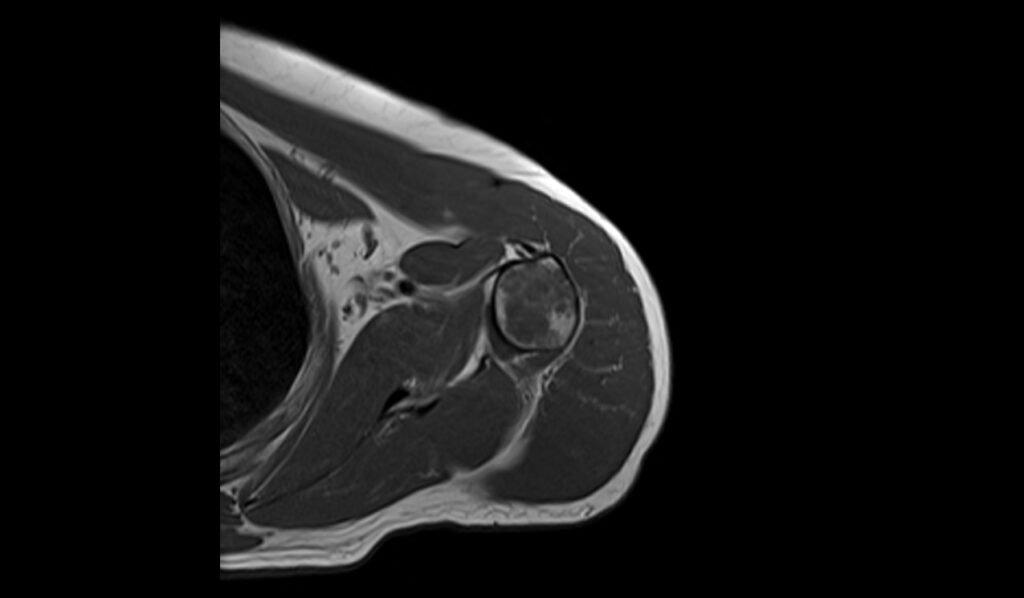
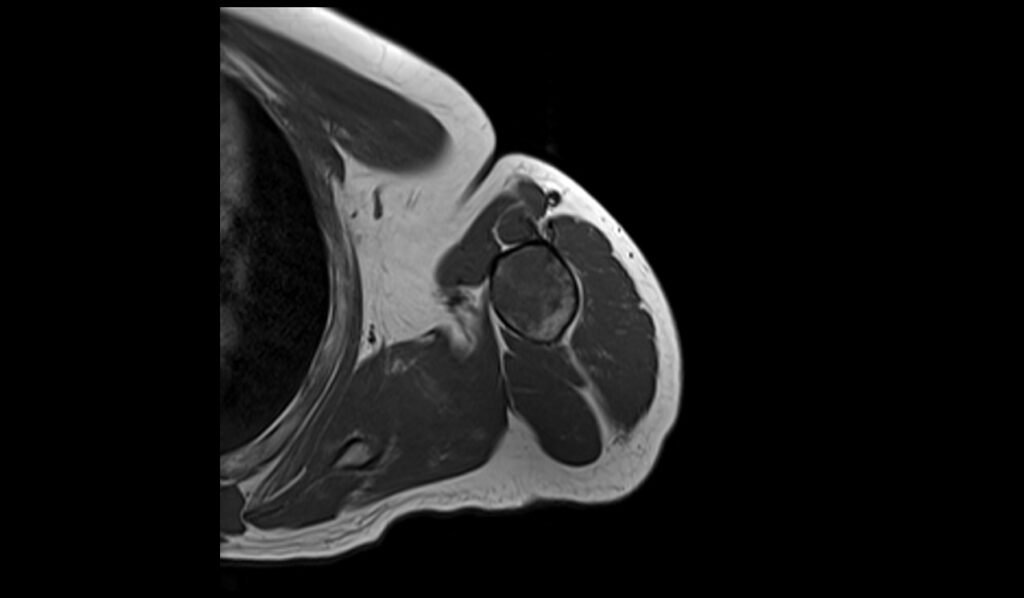
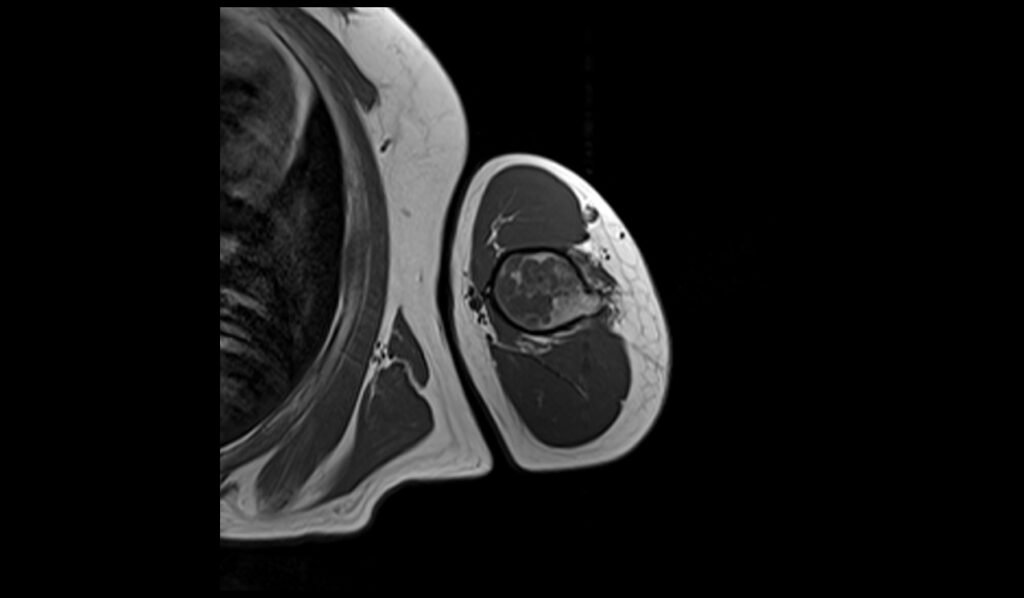
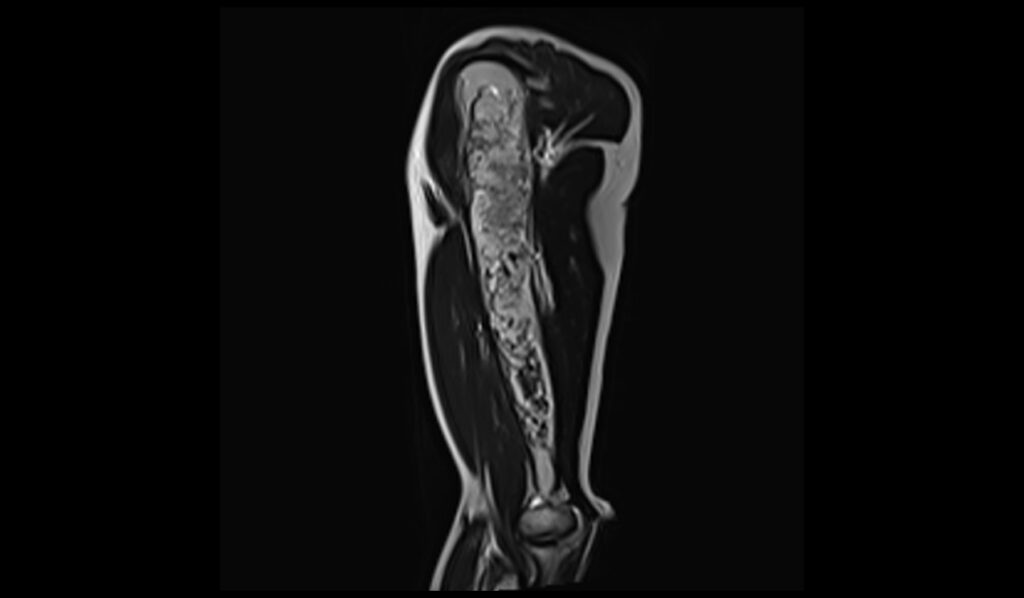
T2 sagittal image shows Humerus Polyostotic Fibrous Dysplasia
References
- Kushchayeva, Y. S., Kushchayev, S. V., Glushko, T. Y., Tella, S. H., Teytelboym, O. M., Collins, M. T., & Boyce, A. M. (2018). Fibrous dysplasia for radiologists: beyond ground glass bone matrix. Insights into Imaging, 9(6), 1035–1056. https://doi.org/10.1007/s13244-018-0666-6.
- Fitzpatrick, K. A., Taljanovic, M. S., Speer, D. P., Graham, A. R., Jacobson, J. A., Barnes, G. R., & Hunter, T. B. (2004). Imaging findings of fibrous dysplasia with histopathologic and intraoperative correlation. American Journal of Roentgenology, 182(6). https://doi.org/10.2214/ajr.182.6.1821389
- Chong, Vincent F. H., James B. K. Khoo, and Yoke-Fun Fan. “Fibrous Dysplasia Involving the Base of the Skull.” American Journal of Roentgenology, vol. 178, no. 3, 2002, https://doi.org/10.2214/ajr.178.3.1780717.


