Glioblastomas
Glioblastomas, also known as malignant gliomas, are aggressive and highly invasive primary brain tumors that pose significant challenges in clinical management. These tumors originate from glial cells, particularly astrocytes, and are characterized by their rapid growth and infiltrative nature. Glioblastomas account for the majority of gliomas and are the most common malignant brain tumors in adults.
Clinically, patients with glioblastomas often present with nonspecific symptoms such as headaches, seizures, cognitive decline, and focal neurological deficits. Radiologically, these tumors appear as heterogeneously enhancing lesions with areas of necrosis and edema on magnetic resonance imaging (MRI). The definitive diagnosis requires histopathological examination, typically through stereotactic biopsy or surgical resection.
Treatment strategies for glioblastomas typically involve a multimodal approach. The standard treatment includes maximal safe surgical resection followed by adjuvant radiotherapy and concurrent chemotherapy with temozolomide, an alkylating agent. Despite aggressive treatment, the prognosis for patients with glioblastomas remains poor, with a median survival of around 15 months.
The challenges in managing glioblastomas stem from their highly invasive nature and resistance to therapies. These tumors infiltrate the surrounding brain tissue extensively, making complete surgical resection nearly impossible.
MRI Appearance Glioblastoma
Glioblastomas display distinct radiological characteristics on magnetic resonance imaging (MRI), playing a crucial role in their identification, evaluation, and management. The MRI findings of glioblastomas have significant implications for diagnosing the condition, planning appropriate treatments, and monitoring disease progression. Here are the key observed features in the MRI of glioblastomas:
T1-weighted images: Glioblastomas typically appear dark (hypointense) on T1-weighted images due to their high cellularity and the presence of necrotic regions. However, after the administration of contrast material, they exhibit heterogeneous enhancement, with certain areas displaying intense enhancement.
T2-weighted and FLAIR images: Glioblastomas appear bright (hyperintense) on T2-weighted and fluid-attenuated inversion recovery (FLAIR) images due to their high water content, presence of necrosis, and edema. The hyperintense signal surrounding the tumor indicates the presence of extensive peritumoral edema.
Contrast enhancement: Glioblastomas typically demonstrate avid and diverse enhancement following the use of gadolinium-based contrast agents. This enhancement pattern reflects disruptions in the blood-brain barrier and increased vascular proliferation within the tumor.
Central necrosis: Glioblastomas often exhibit central necrotic areas, appearing as regions of decreased signal intensity (hypointense or signal void) on T1-weighted images and as bright areas (hyperintense) on T2-weighted/FLAIR images. These necrotic regions frequently exhibit peripheral enhancement.
Mass effect: Due to their invasive nature and the presence of peritumoral edema, glioblastomas can exert a mass effect on adjacent structures. This can lead to the compression of ventricles, midline shift, and displacement of the surrounding brain tissue.
It is important to note that the MRI appearance of glioblastomas may vary depending on factors such as tumor location, size, and molecular characteristics. The interpretation of imaging findings should be done in conjunction with clinical information and histopathological correlation to ensure an accurate diagnosis and appropriate treatment planning.

T2 Axial
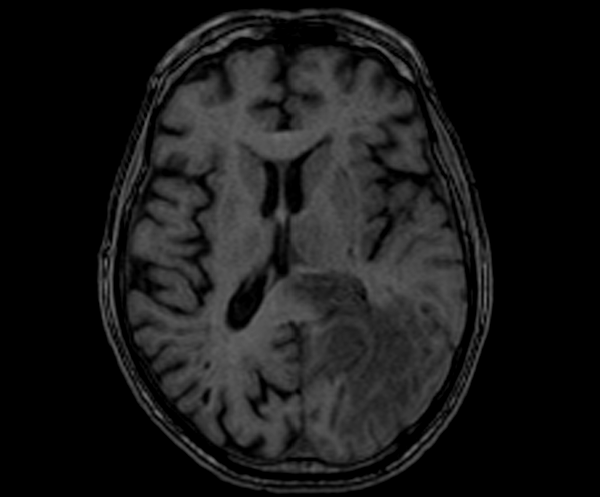
T1 AXIAL PRE CONTRAST

AXI DWI B0
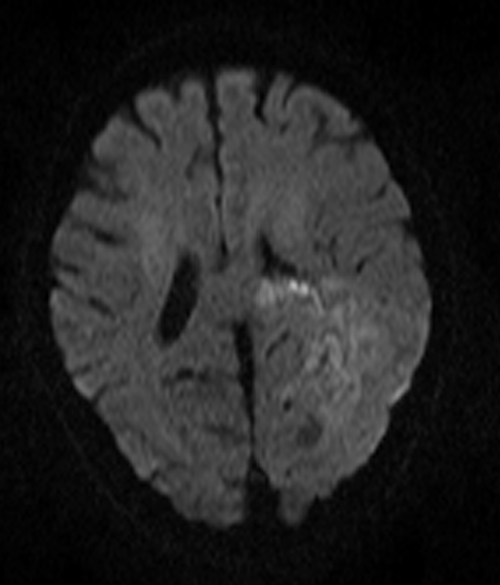
AXI DWI B 1000

AXI DWI ADC
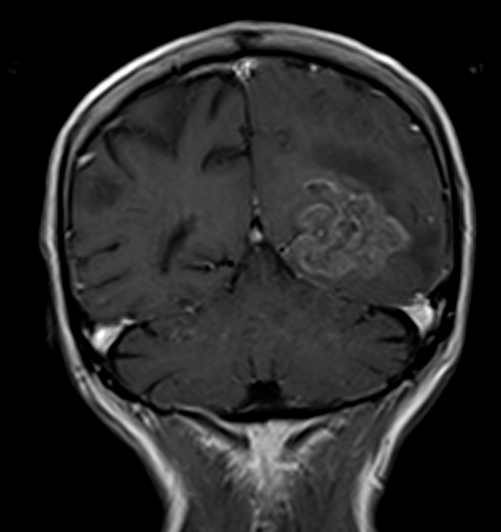
T1 CORONAL POST CONTRAST

T1 AXIAL POST CONTRAST
References
- Stupp, R., Hegi, M. E., Mason, W. P., van den Bent, M. J., Taphoorn, M. J., Janzer, R. C., … & Mirimanoff, R. O. (2009). Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. The Lancet Oncology, 10(5), 459-466.
- Louis, D. N., Perry, A., Reifenberger, G., von Deimling, A., Figarella-Branger, D., Cavenee, W. K., … & Wesseling, P. (2016). The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathologica, 131(6), 803-820.
- Wen, P. Y., & Kesari, S. (2008). Malignant gliomas in adults. New England Journal of Medicine, 359(5), 492-507.
- Ellingson, B. M., Wen, P. Y., & Cloughesy, T. F. (2011). Modified criteria for radiographic response assessment in glioblastoma clinical trials. Neurotherapeutics, 8(4), 629-641.
- Wick, W., Gorlia, T., Bendszus, M., Taphoorn, M., Sahm, F., Harting, I., … & Weller, M. (2017). Lomustine and bevacizumab in progressive glioblastoma. New England Journal of Medicine, 377(20), 1954-1963.
